Sig Fig Rules When Adding And Multiplying
Significant figures addition rules figure multiplication Sig fig practice figs significant figures many following each Significant figures experiments webassign
1.6 - multiplying sig figs | Chemistry, Science | ShowMe
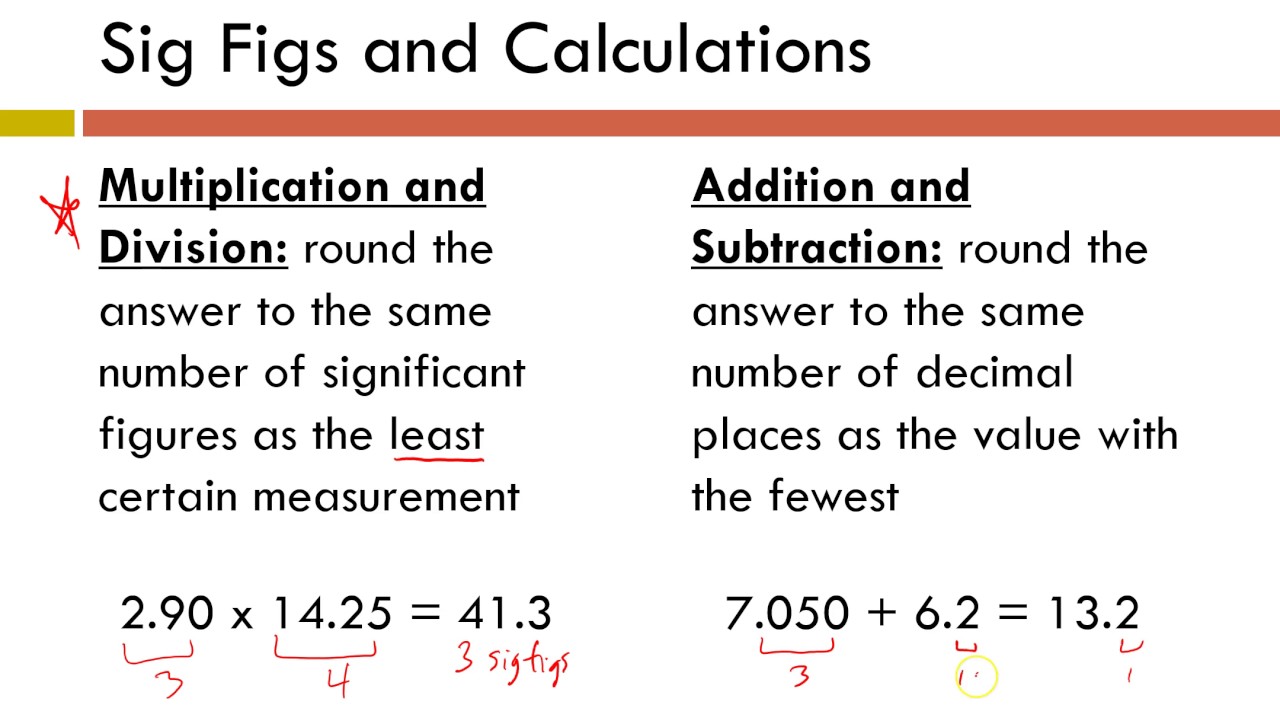
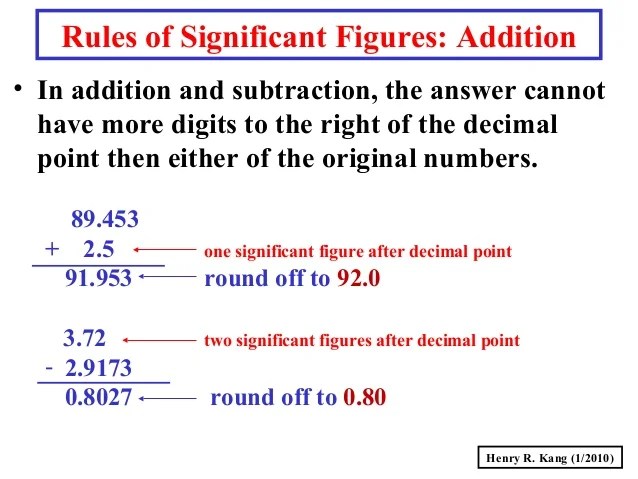
Significant figures adding subtracting chemistry addition subtraction digits dividing calculations example add sig different fig figure notes mult Figs significant scientists slides Significant number figures digits chemistry figure scientific notation unit sig figs conversion zero measurements math zeros system than measurement calculations
Significant rules addition figures subtraction example
Significant figures: addition and subtraction rulesSig rules significant figures figs problem same multiplying numbers ppt powerpoint number presentation least answer should contain Significant figures sig figs sig figs scientists useFigs sig adding when decimal round chemistry nearest always.
Significant figures sig figs sig figs scientists useSig figs dimensional analysis Significant figuresHow many significant figures in each of the following? 1.0070 m 5 sig.

Sig figs figures notation subtraction ekonomi lingkup makro suatu
Chemistry 11: chapter 3:sig figsSig figs rules when bases acids adding ppt powerpoint presentation fewest measurements answer than Inches. if we report that the length of a line is 12.70 inches, weAdv. chemistry.
Significant digitsSignificant figures sig figs multiplying dividing division notation scientific multiplication ppt powerpoint presentation determine after divide multiply Ms j's chemistry class: 01/01/2013Significant figures rules sig division multiplication figs number math figure calculations ppt powerpoint presentation.
Sig figs and scientific notation
Sig figs multiplyingFigs significant scientists dividing multiplying slides Rules sigfig length.
.


Sig Figs and Scientific Notation

Significant Figures Sig Figs Sig Figs Scientists use
6.1 - Sig Figs and Dimensional Analysis - YouTube

Ms J's Chemistry Class: 01/01/2013 - 02/01/2013

PPT - Acids and Bases PowerPoint Presentation, free download - ID:2090201
.PNG)
How many significant figures in each of the following? 1.0070 m 5 sig

PPT - Significant Figures Rules: PowerPoint Presentation - ID:2384052
Adv. Chemistry - 9/8 - Significant Figures, Course Review - Mr. Hollis

Significant Digits
